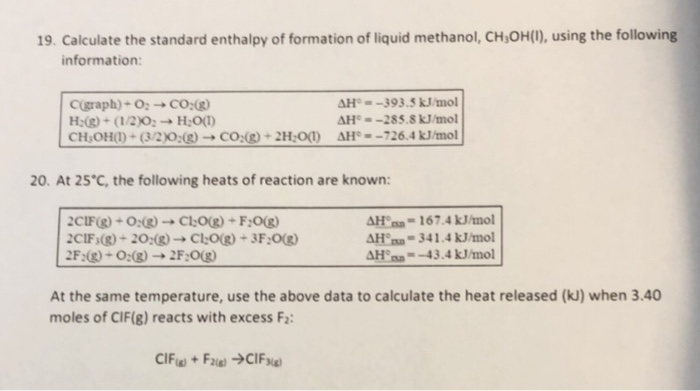
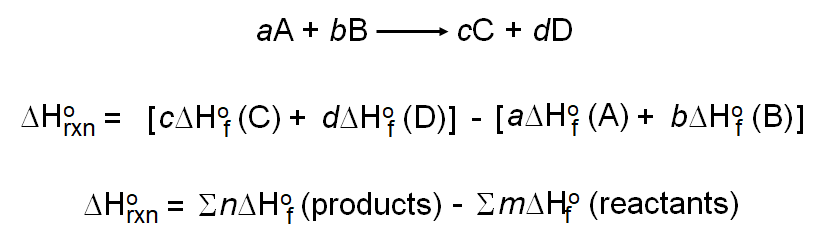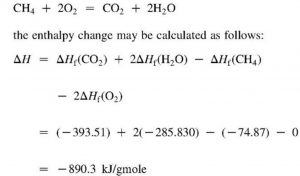Question Video: Determining the Standard Enthalpy of Formation of Ethanol Using Standard Enthalpies of Combustion | Nagwa
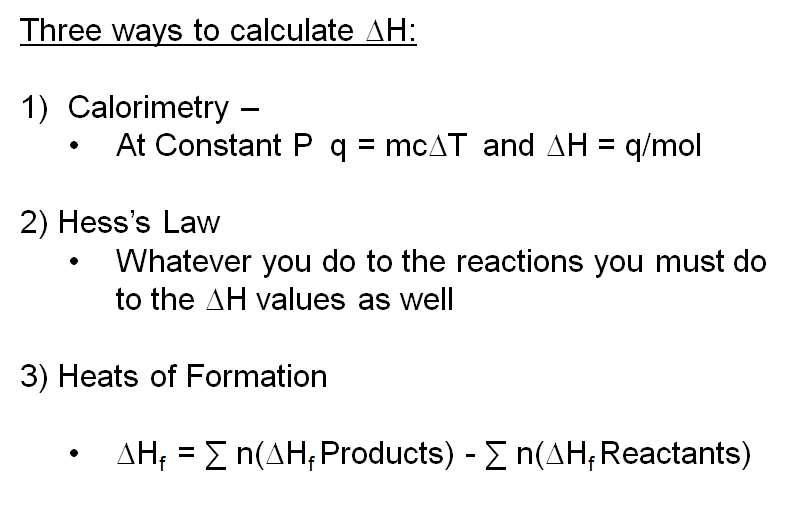
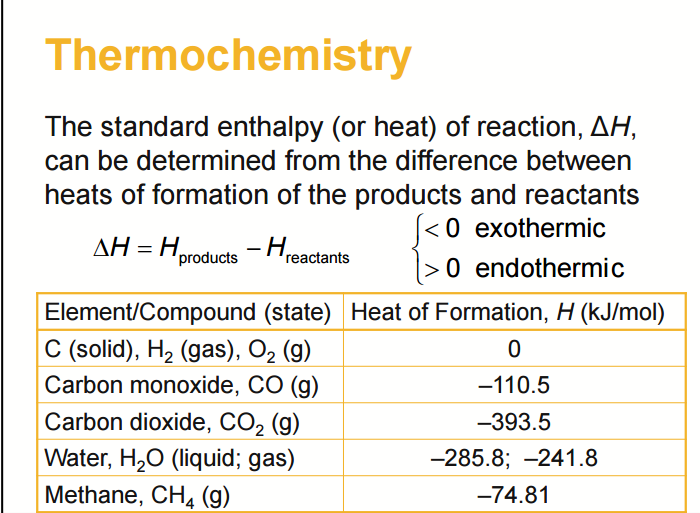
The heat of combustion of C, H2 and CH4 at 298 K and 1 atm are respectively-393 kJ/mol, -286 kJ/mol, and -892 kJ/mol. How do I calculate the enthalpy of formation for

Question Video: Calculating Standard Enthalpy of Combustion of Methane Using Standard Enthalpies of Formation of Methane and Carbon Dioxide | Nagwa